FOR2830
Advanced Concepts in Cellular Immune Control of Cytomegalovirus (FOR 2830)
The human cytomegalovirus (HCMV) persistently infects the majority of the world’s population. While HCMV infection of healthy individuals is usually subclinical, life-threatening HCMV disease is frequent among the immunocompromised. In particular, hematopoietic stem cell or solid organ transplant recipients suffer from HCMV induced pneumonia, colitis, retinitis or graft rejection and transplant failure. An even larger social burden stems from congenital HCMV infection, which clinically affects about 1 in 1,000 newborns. Disease manifestations cover a broad range and variety of structural and functional impairments culminating in long-term neurological sequelae. Clinically relevant HCMV infections arise due to absent or failed immune cell function. Restoring immune cell functions by personalized T cell/NK cell therapies in patients may ensure virus control, yet these strategies so far are not effective in all patients.
The central aim of this RU is to understand the molecular interactions of CMV-infected antigen-presenting cells (APCs) with cytotoxic T cell (CTLs) and natural killer (NK) cells at molecular, cellular and organism level. Studies in the murine cytomegalovirus (MCMV) animal model revealed many fundamental aspects of this interaction that were subsequently confirmed for HCMV. These include but are not restricted to (i) the efficient subversion of CD8 T cell and NK cell responses by viral immune evasins, (ii) the continuous expansion of CMV-specific CD8 T cells (memory inflation) over time, (iii) the intensive interplay between activating and inhibitory NK cell receptors in virus control that shape a host’s susceptibility to CMV infection, and (iv) the generation of ‘memory-like’ NK cells that show features of adaptive immunity. Most recently, studies in the rhesus CMV model have highlighted the great potential of CMV-based vectors for prophylactic and therapeutic vaccination approaches against HIV and other infectious agents.
To account for the growing burden of CMV-associated disease, this RU will generate a structured, collaborative research environment to study cellular immune control of CMV.
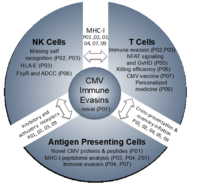
The research unit is divided into the following three subareas:
Subarea 1: MHC-I-mediated recognition of CMV by T cells and NK cells
Subarea 1 (P01 – P03) studies MHC-I-mediated recognition of CMV by T cells and NK cells and viral evasion thereof. P01 aims to determine the full set of HCMV and MCMV gene products/antigens based on the integrative analysis and functional validation of big data. This will include MHC-I presentation of both conventional and non-conventional viral gene products (Z01). P02 focuses on how viral genes that modulate MHC-I and the MHC-I-ligandome counteract both CD8 T cell killing and NK cell missing-self recognition but subsequently trigger activating NK cell receptors. Vaccination of rhesus macaques with a rhesus CMV with restricted cell tropism resulted in vigorous CD8 T cell responses to peptides presented by MHC-E (7). P03 explores cell type-specific differences in HLA-ABC- and HLA-E-ligandomes, to clarify the role of HLA-E-restricted epitopes in HCMV infection and study a novel HCMV HLA-E modulator (P03).
Subarea 2: Mechanisms defining T cell and NK cell activation and function
Subarea 2 (P04 – P06) studies key aspects of T cell activation and control of CMV infection in conditions of natural infection and immunosuppression. P04 investigates peptides from conventional and non-conventional viral gene products for TAP-dependent and TAP-independent presentation on APCs under conditions of direct- or cross-presentation. P05 is based on the interesting observation that inflation but not the induction of a conventional CD8 T cell memory response depends on NFAT signaling. Hence, it analyses CD8 T cell responses to MCMV in general and after allogeneic stem cell transplantation with various NFAT-deficient mice in order to clarify the potential of NFAT-deficient CD8 T cells in controlling MCMV while avoiding graft-vs-host disease (GvHD). P06 aims to analyze, assess and optimize the killing efficiency of CD8 T cells recognizing canonical and non-canonical epitopes using advanced imaging techniques (2-photon microscopy).
Subarea 3: Improving immunological control of CMV for clinical interventions
Subarea 3 (P07 – P09) aims to develop innovative approaches for immune intervention. P07 aims to decipher and exploit the improved efficacy of HCMV-based vaccine vectors expressing stress-inducible co-stimulatory molecules (ULBP1, -2, MICA, MICB) and assess the interplay between different stress-induced ligands and HLA-I in both T and NK cell activation. P08 assesses the diagnostic and therapeutic potential of HCMV-immune IgG as activator of FcγR-mediated immune effector functions including NK cell-mediated ADCC using a newly developed reporter cell system. This includes targeting HCMV-encoded FcγR antagonists that interfere with antiviral IgG-binding to FcγRIII/CD16 on NKG2Cbright NK cells and thereby impair ADCC. Finally, we aim to exploit and translate our findings from (i) revised HCMV genome annotation (P01), (ii) HLA-I ligandome analyses (Z01) including HLA-E (P03), (iii) optimized antigen presentation (P04) and co-stimulation (P07), and (iv) advanced imaging studies (P06) to develop new diagnostic, prophylactic and therapeutic HCMV-specific T cell approaches based on findings of the other subareas and projects at UKW and MHH (P09).